how to draw molecular orbital diagram khan academy
P option enables extended printout popfull enables. General Notes on Molecular Orbital Diagrams.

Ap Chemistry Mo To Band Theory Youtube
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.
. Sal Khan Courses 498 View detail Preview site. Shape of Orbitals in Molecules. Any additional bonds come from electrons in p-orbitals.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Type P popfull GFInput in Gaussian input files. Individual atomic orbitals AO are arranged on the far left and far right of the diagram.
He also discusses applications in understanding reactions and in UVvisible absorption s. Open the output file produced by popular quantum-chemical calculation programs USGamess PCGamess Gaussian Q-Chem Spartan containing molecular orbitals data. Using this resource you can add pieces to pre-drawn MO diagrams for over 20 different molecules.
It covers the basics of how to solve for bond order. 3 days ago Dec 13 2019 The electrons in an atom are arranged in shells that surround the nucleus with each successive shell being farther from the nucleus. Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules.
The site includes opportunities to practice filling in electrons attaching the namessymbols of MOs and matching. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions.
Written By Deering Befiscure Tuesday 19 April 2022 Add Comment Edit Should I Sysprep A Vmware Template. AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in. 2 Molecular Orbitals of NH3 C3v.
1 week ago Oct 06 2019 1. In order to figure out the shape that electron orbitals make in a molecule you need to look at the shape of the orbitals in the atoms and how they combine and. The first key part of this is to understand which orbitals interact with each other.
Construct a qualitative molecular orbital diagram for chlorine Cl 2. The HOMO and LUMO. See Resources for a diagram showing the filling order.
For atoms like nitrogen the lack of electron. In molecular orbital theory a covalent bond is formed whenever two atoms overlap all of their orbitals regardless of whether they are valence orbitals or not to. I need some help drawing a NH3 molecular orbital.
One of the greater problems I have is that I cant find MO in LaTeX which are so complex as my diagram. The intuition of bond order orbital conf. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram.
Shells subshells and orbitals video Khan Academy. Electron shells consist of one or more subshells and subshells. Electrons in an anti-bonding orbital will weaken the bonds of the molecule.
Compare the bond order to that seen in the Lewis structure remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a. By Marsha Massey. This is a very basic introduction to molecular orbital theory.
In MO theory explaining bonding anti bonding and non bonding orbitals in general and how to fill the electrons in the orbitals. The Y-axis of a MO diagram represents the total energy not potential nor Gibbs Energy of the orbitals. Essentially the orbitals which interact have to have the same symmetry.
I would also greatly appreciate a series on this topic. Single bonds come from electrons in s-orbitals. University of Sydney has created a practice website for reviewing different parts of molecular orbital diagrams.
Norris discusses frontier molecular orbitals. Next well see that symmetry will help us treat larger. Molecular orbital theory - Khan Academy Help Center.
So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. An orbital correlation attempts to show how the atomic orbitals belonging to the individual atoms in a molecule interact to form the molecular orbitals. In molecular orbital theory a covalent bond is formed whenever two atoms overlap all of their orbitals regardless of whether they are valence orbitals or not to create bonding and antibonding orbitals.
This can get to be a really. The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize. Click File-Open menu and select the file containing molecular orbitals data Note to the Gaussian users.
Yes this is found in p subshells when forming homonuclear molecules with some atoms. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals. Between molecules like N2 O2 and others like HF.
In this section were going to learn how to draw and utilize. Asana-created templates Skip. It is recommended to name the SVG file.
Electrons in bonding orbitals will strengthen the bonds of the molecule. Lewis VSEPR Valence Orbitals and MO.
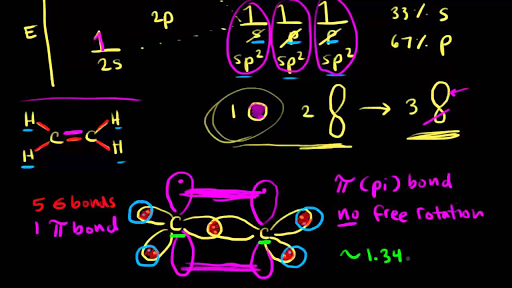
Chemical Bonding And Molecular Structure Khan Academy

Orbital Diagram Of Carbon Teaching Chemistry Chemistry Lessons Science Chemistry

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Drawings Tech Company Logos Lewis
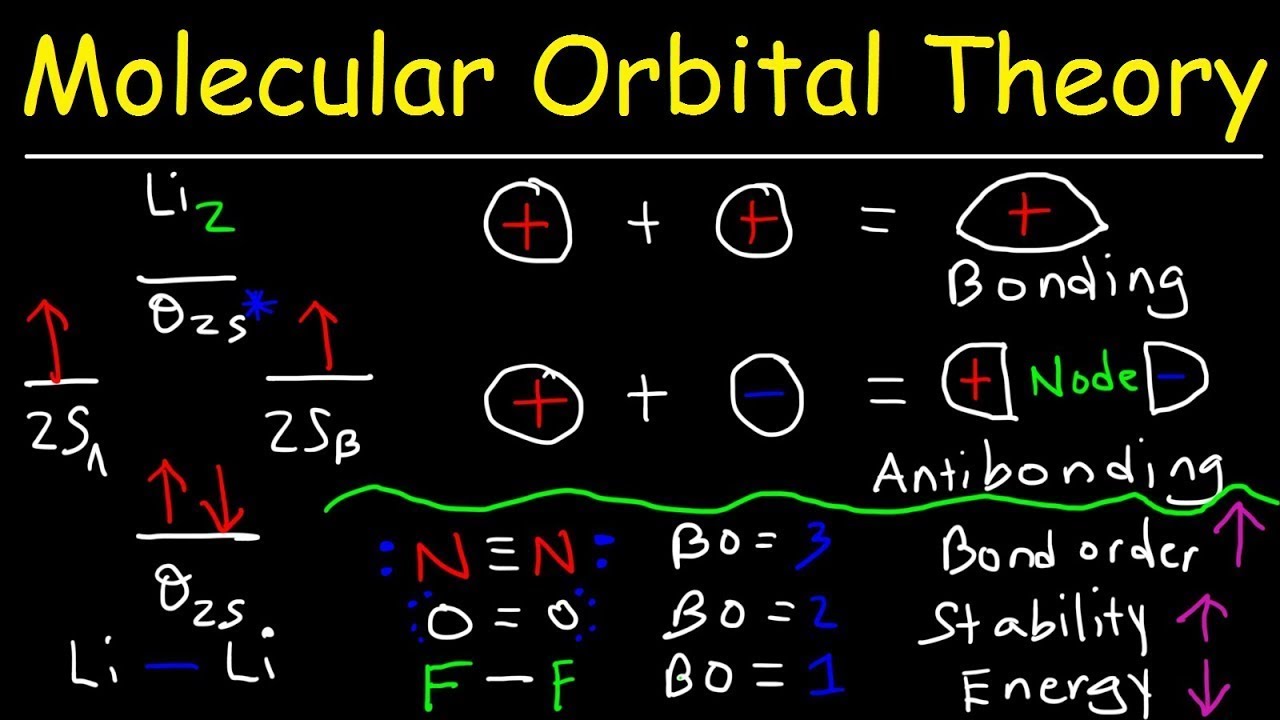
Molecular Orbital Theory Bonding Antibonding Mo Bond Order Youtube

Tom Teoria Do Orbital Molecular Diagram Electron Configuration Ionization Energy

This Is A 14 Page Guided Notes Packet Used To Explain Some Of The Fundamental Concepts In The Development Of Atomic Structu Ap Chemistry Guided Notes Chemistry

What Is An Sp3 Hybridized Carbon Atom A Plus Topper Sp3hybridization Atom Carbon Chemistry

Catalyst Speeds Up A Chemical Reaction By Lowering The Activation Energy Ea Of The Overall Reaction Chemistry Education Teaching Chemistry Energy Activities

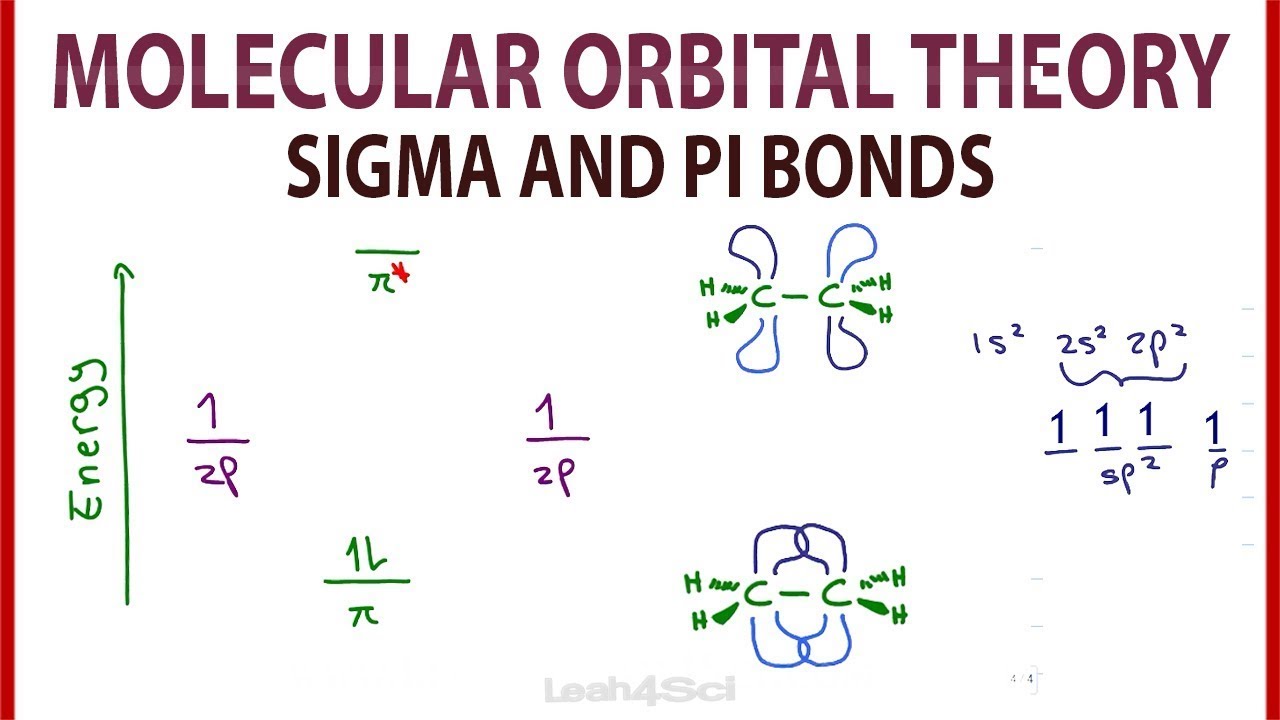
Molecular Orbital Mo Theory Simplified For Sigma And Pi Bonds Youtube

Standard Electrode Potential Google Search Reduction Potential Reducing Agent Science And Technology

Chemistry 101 Molecular Orbital Theory Youtube
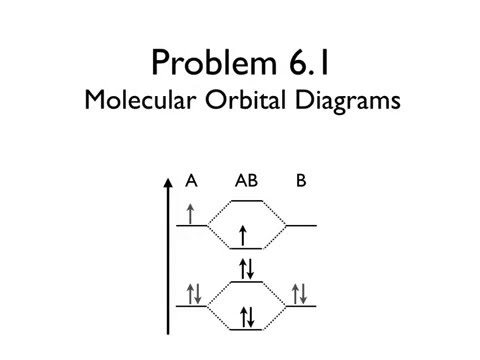
Molecular Orbital Diagrams Chemistry X Youtube

Catalyst Speeds Up A Chemical Reaction By Lowering The Activation Energy Ea Of The Overall Reaction Chemistry Education Teaching Chemistry Energy Activities

Types Of Isomers Structural Isomer Stereoisomer Molecular Geometry Structural Formula Molecular

Fischer Projections Practice Problems Practice Chemistry Organic Chemistry

Orbital Diagrams And Electron Configuration Basic Introduction Chemistry Practice Problems Youtube
The Aufbau Principle Video Khan Academy

Pin By Cristina Covalciuc On Neuro Anatomy And Physiology Brain Art Neuro
